Hospital Administrators
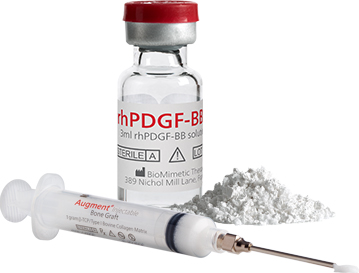 AUGMENT® Regenerative Solutions is the first and only proven alternative to autograft in ankle and hindfoot arthrodesis. Available in two delivery options, AUGMENT® Bone Graft is a combination of recombinant human platelet derived growth factor BB (rhPDGF-BB), that contributes to mesenchymal stem cell chemotaxis and mitogenesis and angiogenesis; and Beta tri-calcium phosphate (β-TCP) granules, that provide an osteoconductive scaffold to facilitate new bone formation and delivery of rhPDGF-BB. AUGMENT® Injectable includes the same rhPDGF-BB and (β-TCP) as found in AUGMENT® Bone Graft, but it also includes a collagen matrix that allows AUGMENT® to be flowable.
AUGMENT® Regenerative Solutions is the first and only proven alternative to autograft in ankle and hindfoot arthrodesis. Available in two delivery options, AUGMENT® Bone Graft is a combination of recombinant human platelet derived growth factor BB (rhPDGF-BB), that contributes to mesenchymal stem cell chemotaxis and mitogenesis and angiogenesis; and Beta tri-calcium phosphate (β-TCP) granules, that provide an osteoconductive scaffold to facilitate new bone formation and delivery of rhPDGF-BB. AUGMENT® Injectable includes the same rhPDGF-BB and (β-TCP) as found in AUGMENT® Bone Graft, but it also includes a collagen matrix that allows AUGMENT® to be flowable.
AUGMENT® Bone Graft was Approved by the FDA through the rigorous PMA process, reserved for unprecedented, pioneer products. As a result of the largest study conducted in foot and ankle history to date, AUGMENT® was proven equivalent to autograft in clinical outcomes but without any donor site pain.
Fast Facts
- The only Class III US FDA-approved alternative to autograft bone for fusion procedures of the ankle and hindfoot.1
- Rigorously studied in over 500 patients, across 4 human clinical trials conducted in the US and Canada including the largest Level 1 clinical trial ever conducted in the foot and ankle.2,3,4
- Approved and safely used in Canada beginning in 2009 and in Australia and New Zealand beginning in 20112,5,6,7,8,9 in foot and ankle fusions or surgeries.
- Clinically proven to offer comparable clinical success to the gold standard of autograft bone in foot and ankle fusion procedures.2
- Spares patients all risk for complications, postoperative morbidity, increased surgical time, longer hospital stays, short- and long-term impact on patient quality of life, and interference with routine activities of daily living associated with autograft bone harvest.6,10,11,12,13,14,15,16,17,18,19
- The rhPDGF-BB biologic component is engineered via recombinant DNA technology, as an exact replica of endogenous human PDGF-BB, thus eliminating risk of disease transmission or immune response possible with allogeneic bone.5– rhPDGF-BB triggers the tissue healing and bone repair cascade.
- The β-TCP matrix component allows targeted delivery of the rhPDGF-BB, fills the bone defect, provides an osteoconductive matrix, prevents soft tissue prolapse, and provides the environment necessary for the stability of a newly forming callus at the wound healing site.20,5,21
– β-TCP is resorbed and replaced with bone during healing. - AUGMENT® offers surgeons and patients a better way to achieve fusion, by eliminating the need for harvesting autograft bone.
- Surgeons are in general agreement regarding clinical and radiographic indications for requiring bone graft in F/A fusion surgery.22
- Nonunion after foot and ankle fusion procedures is indicative of poorer functional outcomes and quality of life.23
- Improved fusion results on CT are associated with improved clinical outcomes.*24
- Using graft material in sufficient quantities provides substantive value in maximizing the likelihood of joint fusion and promoting successful clinical union.25
Drawbacks to Autograft Bone Harvest
- There is a finite amount of bone that can be safely and reasonably harvested.5,26
- The amount of bone available to be harvested may be insufficient, depending on the size of the bone defect to be treated at the primary surgical site, the physical size of the patient, the number of joints to be fused.5
- The presence of comorbidities such as osteoporosis can even further reduce the quantity of bone available for harvest.5,26
- Risk of nonunion during foot and ankle fusion surgery is of clinical concern to surgeons.5,26
- Some foot and ankle experts suggest that supplementation of foot and ankle fusion procedures should be considered for all patients.12
Autograft Bone Harvest is Not Free
The costs associated with bone graft harvest can be substantial when operating room time, instrument costs, anesthesia costs, physician fees, and management of postoperative complications are taken into account:
- Harvesting bone graft may add additional time to the operating room time for the overall fusion procedure.5,6,19
- Addressing associated complications from bone graft harvest, may add up to 2 additional days to the hospitalization length of stay.5,15,19,27
- Harvesting bone graft material can slow recovery time leading to lower levels of patient satisfaction, affect activities of daily living, and delay the return to work.28
- There is clinical value in sparing patients of the morbidity associated with the autograft harvest procedure in foot and ankle fusion surgery. Chronic pain is reported at the graft harvest sites, including proximal tibia, distal tibia, calcaneus, and iliac crest.29
*FDA did not base its approval of AUGMENT® on radiologic findings from the pivotal study, but instead relied on clinical outcomes.
References:
2. DiGiovanni CW, Lin SS, Baumhauer JF, Daniels T, Younger A, et al. Recombinant human platelet-derived growth factor-BB and beta-tricalcium phosphate (rhPDGF-BB/B-TCP): an alternative to autogenous bone graft. J Bone Joint Surg Am 95: 1184-92 [2013].
3. Daniels T, DiGiovanni C, Lau JT, Wing K, Younger A. Prospective clinical pilot trial in a single cohort group of rhPDGF in foot arthrodeses. Foot Ankle Int 31(6): 473-9 [2010].
4. DiGiovanni CW, Baumhauer J, Lin SS, Berberian WS, Flemister AS, et al. Prospective, randomized, multi-center feasibility trial of rh PDGF-BB versus autologous bone graft in a foot and ankle fusion model. Foot Ankle Int 32(4): 344-354 [2011].
5. DiGiovanni CW, Lin S, Pinzur M. Recombinant human PDGF-BB in foot and ankle fusion. Expert Rev Med Devices 9 (2): 111-22 [2012].
6. Geideman W, Early JS, Brodsky J. Clinical results of harvesting autogenous cancellous graft from the ipsilateral proximal tibia for use in foot and ankle surgery. Foot Ankle Int 25 (7): 451-455 [2004].
7. Australian Government, Department of Health, Therapeutic Goods Administration. http://apps.tga.gov.au/prod/DEVICES/daen-entry.aspx
8. Health Canada. http://webprod3.hc-sc.gc.ca/arquery-rechercheei/report-rapport-elements.do?lang=eng
9. New Zealand Medicines and Medical Devices Safety Authority. http://www.medsafe.govt.nz/hot/Recalls/RecallSearch.asp
10. O’Keefe RM, Riemer BL, Butterfield SL. Harvesting of autogenous cancellous bone graft from the proximal tibial metaphysis: a review of 230 cases. J Orthop Trauma 5(4): 469-474 [1991].
11. Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity: a statistical evaluation. Spine 20: 1055-1060 [1995].
12. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop 32: 300-309 [1996].
13. Goulet JA, Senunas LE, DeSilva GL, Greenfield ML. Autogenous iliac crest bone graft complications and functional assessment. Clin Orthop 339: 76-81 [1997].
14. Schulhofer DS, Oloff LM. Iliac crest donor site morbidity in foot and ankle surgery. J Foot and Ankle Surgery 36 (2): 155-158 [1997].
15. St. John TA, Vaccaro AR, Sah AP, Schaefer M, Berta SC, et al. Physical and monetary costs associated with autogenous bone graft harvesting. Am J Orthop 32 (1): 18-23 [2003].
16. DeOrio JK, Farber DC. Morbidity associated with anterior iliac crest bone grafting in foot and ankle surgery. Foot Ankle Int 26 (2): 147-151 [2005]
17. Raikin SM, Brislin K. Local bone graft harvested from the distal tibia or calcaneus for surgery of the foot and ankle. Foot Ankle Int 26:449-453 [2005]
18. Chou LB, Mann RA, Coughlin MJ, McPeake WT, Mizel MS. Stress fracture as a complication of autogenous bone graft harvest from the distal tibia. Foot Ankle Int 28(2): 199-201 [2007].
19. Lohmann H, Grass G, Rangger C, Mathiak G. Economic impact of cancellous bone grafting in trauma surgery. Arch Ortho Trauma Surg 127, 345- 348 [2007].
20. Solchaga LA, Hee CK,Roach S, Snel LB. Safety of recombinant human platelet-derived growth factor-BB in Augment® Bone Graft. Journal of Tissue Engineering 3(1). [2012].
21. Solchaga LA, Daniels T, Roach S, Beasley W, Snel LB. Effect of implantation of AUGMENT® Bone Graft in serum concentrations of platelet-derived growth factors: a pharmacokinetic study. Clinical Drug Investigation 33: 143-149. [2013].
22. Baumhauer J, et al. Survey on the need for bone graft in foot and ankle fusion surgery. Foot Ankle Int 34(12): 1629-33. [2013].
23. Younger ASE, et al. Clinical outcomes of nonunions of hindfoot and ankle fusions. J Bone Joint Surg Am. 98A(23): 2006-16. [2016].
24. Glazebrook M, et al. Establishing the relationship between clinical outcome and extent of osseous bridging between computed tomography assessment in isolated hindfoot and ankle fusions. Foot Ankle Int. 34(12): 1612-8. [2013].
25. DiGiovanni CW, et al. The importance of sufficient graft material in achieving foot or ankle fusion. J Bone Joint Sur Am. 98(15): 1260-7. [2016].
26. DiGiovanni CW, Petricek JM. The evolution of rhPDGF-BB in musculoskeletal repair and its role in foot and ankle fusion surgery. Foot Ankle Clin N Am 15, 621-40 [2010].
27. Dahabreh Z, Calori GM, Kanakaris NK, Nikolaou VS, Giannoudis PV. A cost analysis of treatment of tibial fracture nonunion by bone grafting or bone morphogenetic protein-7. Int Orthopaedics 33: 1407-1414 [2009].
28. Abidi NA, Carlson AM, Harris EM. An Analysis of Cost of Autologous Bone Graft. 2012 American Orthopaedic Foot and Ankle Society Annual Summer Meeting: San Diego, CA, June 20-23, 2012.
29. Baumhauer J, et al. Site Selection and pain outcome after autologous bone graft harvest. Foot Ankle Int. 35(2): 104-7. [2014].